Heavy Rare Earths
What are Rare Earths?
High-operating temperature ‘HOT’ magnets require Heavy REEs ‘Dy’ and ‘Tb’
The magnets necessary in EV and military applications
There are two types of REEs
What are Heavy Rare Earths
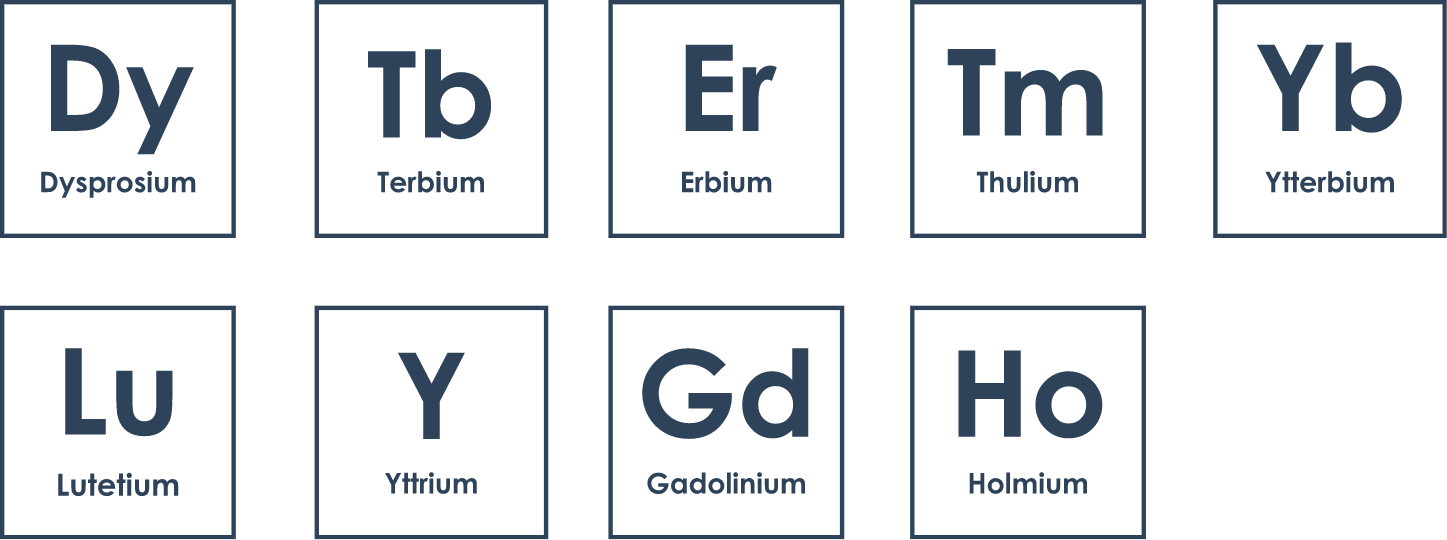
Heavy rare earth metals are defined by their higher atomic weights relative to light rare earths. They are less common, and some elements within the group are facing shortages as demand outpaces supply. That typically makes them more valuable than light rare earths, though they also have smaller markets. The full list of heavy rare earths is dysprosium, yttrium, terbium, holmium, erbium, thulium, ytterbium, yttrium and lutetium.
Dysprosium, yttrium and terbium are considered critical in the heavy rare earth metals group as they face low supply and increasing importance in the development of clean energy technologies. Like the light rare earths, heavy rare earths also play a key role in other technology, including hybrid cars, fiber optics and medical devices.
Dysprosium is used in tandem with neodymium in magnets that are vital to modern tech and renewable energy. In addition, dysprosium oxide is used in nuclear reactors to help cool fuel rods to keep reactions under control.
What are Light Rare Earths


All rare earth metals are part of a chemical group called lanthanides, and light rare earths represent the lanthanides with the lowest atomic numbers.
Of the light rare earth metals, neodymium is considered one of the most critical. It is used in everything from mobile phones and electric cars to medical equipment. Neodymium is also the main light rare earth used in the creation of permanent magnets, which are heavily used in data storage systems and wind turbines.
Praseodymium is another significant light rare earth metal. It is used in alloys with magnesium to form aircraft engines, and it also finds use in the film industry for studio lighting and other projects. Like many rare earth metals, praseodymium plays a role in creating permanent magnets.
Rare earth elements comprise 15 lanthanides plus Scandium and Ytterbium
What is Dysprosium?
As a member of the heavy-group rare-earth elements (HREE), dysprosium has two paired electrons giving it the ability to detect radiation, improve permanent magnets, store digital data, precisely aim lasers, emit sonar pings, or glow in the dark.

Atomic Symbol: Dy
Atomic Number: 66
Element Category: Lanthanide metal
Atomic Weight: 162.50
- Dysprosium in Terfenol-D, is used to produce sonar sensors, positioning actuators, active noise and vibration cancellation, seismic waves, and tool machining.
- A dysprosium additive to neodymium-iron-boron magnets increases the operating temperature range for use in hybrid and electric vehicles.
- Dysprosium phosphide (DyP) is a semiconductor used in laser diodes and high power, high-frequency applications.
- Dysprosium oxide in a cermet is used in nuclear reactor control rods to control the fission process.
- Dysprosium-165 is injected into joints in the body to treat rheumatoid arthritis.
- Radiation badges to detect and monitor radiation exposure.
- Coating compact disks (CD) for digital data, music, and video storage.
Dysprosium was discovered by French chemist Paul Émile Lecoq de Boisbaudran in 1886. Working with an impure holmia, Lecoq de Boisbaudran used fractional crystallisation to separate the impure holmia using ammonium hydroxide, followed by additional separations using potassium sulfate. After multiple fractionations, four “earths” precipitated in the following order: terbium, dysprosium, holmium, and erbium. Three of the elements had previously been discovered. In discovering dysprosium, Lecoq de Boisbaudran noted that he had very little material to work with and confided in Professor Georges Urbain that most of his fractional crystallisations had been prepared on a marble fireplace slab at his residence in Cognac, France. Dysprosium is named after the Greek word, dusprositos (δυσπροσιτός), meaning difficult to approach or get at, in reference to the painstaking number of fractional crystallisations needed to make the precipitate.
Large resources of dysprosium in xenotime and monazite are available worldwide in ancient and recent placer deposits, uranium ores, and weathered clay deposits (ion-adsorption ore). It occurs in the Earth’s crust at an average concentration of 3 parts per million(ppm). Xenotime is enriched in dysprosium oxide and contains 8% to 9% of the rare-earth oxide (REO) content. Monazite-(Ce), which is more abundant in the Earth’s’ crust than xenotime, has dysprosium oxide contents of 0.2% to 0.9% of the REO content.
Hastings’ Yangibana Project contains an average of around 50ppm Dy2O3, which is higher than the average concentration in the earth’s crust.
What is Terbium?
Terbium is a silvery-white, rare earth metal that is malleable, ductile, and soft enough to be cut with a knife. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogengas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime, and euxenite.

Atomic Symbol: Tb
Atomic Number: 65
Element Category: Lanthanide metal
Atomic Weight: 158.925
Used as a dopant in calcium fluoride, calcium tungstate, and strontium molybdate, materials that are used in solid-state devices, and as a crystal stabilizer of fuel cells which operate at elevated temperatures, together with ZrO2.
Used in alloys and in the production of electronic devices. As a component of Terfenol-D, terbium is used in actuators, in naval sonar systems, sensors, in the SoundBug device (its first commercial application), and other magnetomechanical devices. Terfenol-D is a terbium alloy that expands or contracts in the presence of a magnetic field. It has the highest magnetostriction of any alloy.
Used in green phosphors in fluorescent lamps and color TV tubes. Sodium terbium borate is used in solid state devices. The brilliant fluorescence allows terbium to be used as a probe in biochemistry, where it somewhat resembles calcium in its behavior. Terbium “green” phosphors (which fluoresce a brilliant lemon-yellow) are combined with divalent europium blue phosphors and trivalent europium red phosphors to provide the trichromatic lighting technology which is by far the largest consumer of the world’s terbium supply. Trichromatic lighting provides much higher light output for a given amount of electrical energy than does incandescent lighting.
Used to detect endospores, as it acts as an assay of dipicolinic acid based on photoluminescence
Terbium was first isolated in 1843 by the Swedish chemist Carl Mosander at Stockholm. He had already investigated cerium oxide and separated a new element from it, lanthanum, and now he focussed his attention on yttrium, discovered in 1794, because he thought this too might harbour another element. In fact Mosander was able to obtain two other metal oxides from it: terbium oxide (yellow) and erbium oxide (rose pink) and these he announced in 1843. This was not the end of the story, however, because later that century these too yielded other rare earth elements (aka lanthanoids). Today these elements are easily separated by a process known as liquid-liquid extraction.
Along with other rare earth elements, terbium can be found in minerals, including cerite and gadolinite. The element can be extracted from monazite, in which it is present to the extent of 0.03 percent; from euxenite, a complex oxide containing 1 percent or more of terbia; and xenotime.
Recent advances ion-exchange techniques for separating the rare earth elements have enabled the isolation of terbium. One method for producing the rare earth metal is by reducing the anhydrous chloride or fluoride with calcium, although other methods of isolation are available. Vacuum remelting can remove calcium and tantalum impurities.
REEs are in demand for a variety of uses

Including high-end military defence applications

Visors &
Protection
Anti-Missile
Defence
Missile guidance
systems

GPS
Aircraft parts
& jet engines

Communication
Permanent Magnets represent the greatest demand for REEs
Permanent magnets are critical for the ‘Energy Transformation’
- Used in generators for wind turbines – enable wind turbines to reduce overall weight, cost of other components, maintenance costs and improves reliability and efficiency.
- Used in traction motors for electric vehicles – outstanding properties give greater efficiency and range.
- Increase motor strength and reduce energy consumption of home appliances and air conditioning units.
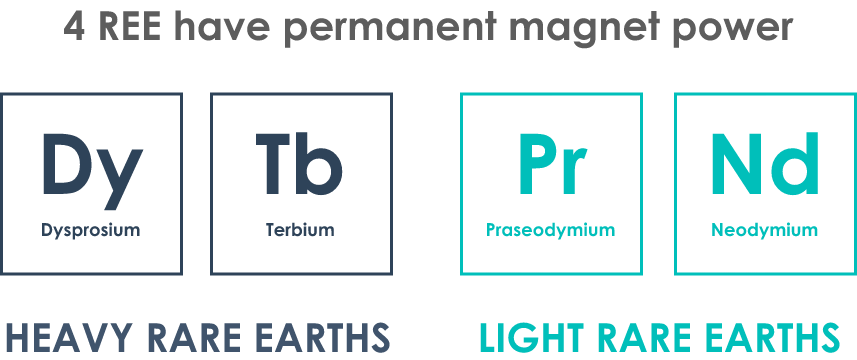
The importance of heavy rare earths (HREE)
HREE are needed in order to complement LREE in a high-performance magnet
– Dy & Tb are used to improve a magnet’s resistance to demagnetization, allowing for higher working temp
– EV motor operating temperature varies between 180°C to 240°C
Heavy REEs Dy & Tb in high demand
The move towards electrification and decarbonisation is undoubted – permanent magnets play a critical role which creates strong long-term demand for key magnet minerals
Dy and Tb are critical minerals in the supply chain for high end magnet uses

Batteries are critical as they store energy, but permanent magnets transform that energy into kinetic energy
Permanent magnets and catalysts responsible for ~65% of global RE demand by volume, and permanent magnets only, ~95% by value (in 2021)1
Value of magnet RE market is expected to triple by 20351
Dy and Tb are key components in the manufacture of performance technology solutions for clean energy
High-end uses such as wind turbines and EV motors are expected to have higher demand growth
Not enough Dy and Tb to source projected demand forecasts
By 2035, expectation of a shortage equal to 2-3x 2021 global output1
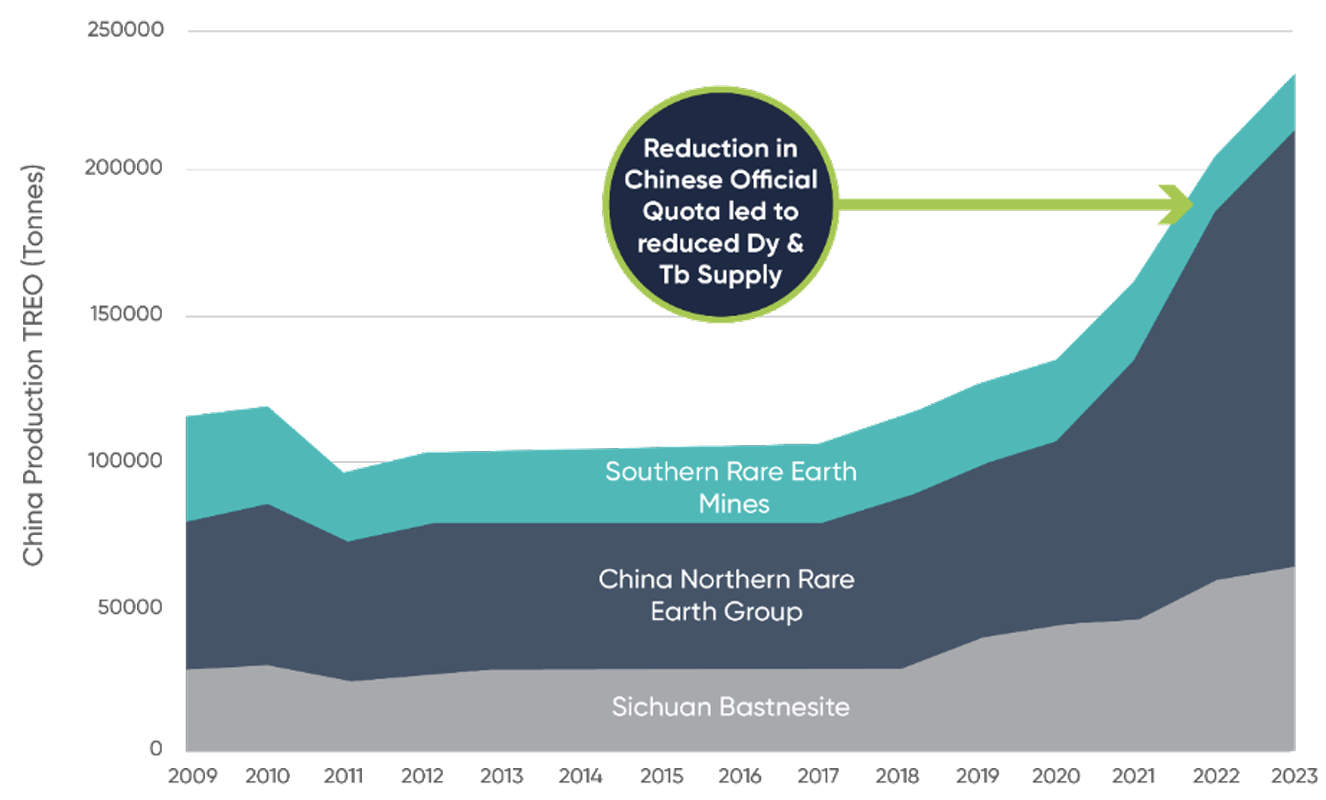
Current geographical concentration supports creating an ex-China supply chain for these high-end magnetic uses
China and Myanmar currently responsible for >90% of global Dy/Tb mine production and >99% is refined in China1
1. Source: Adamas Intelligence: Rare Earth Magnet Market Outlook to 2035 at Q2 2022
ADDITIONAL STABLE HREE SUPPLY REQUIRED TO REDUCE DEPENDENCY
DYSPROSIUM AND TERBIUM PRICES
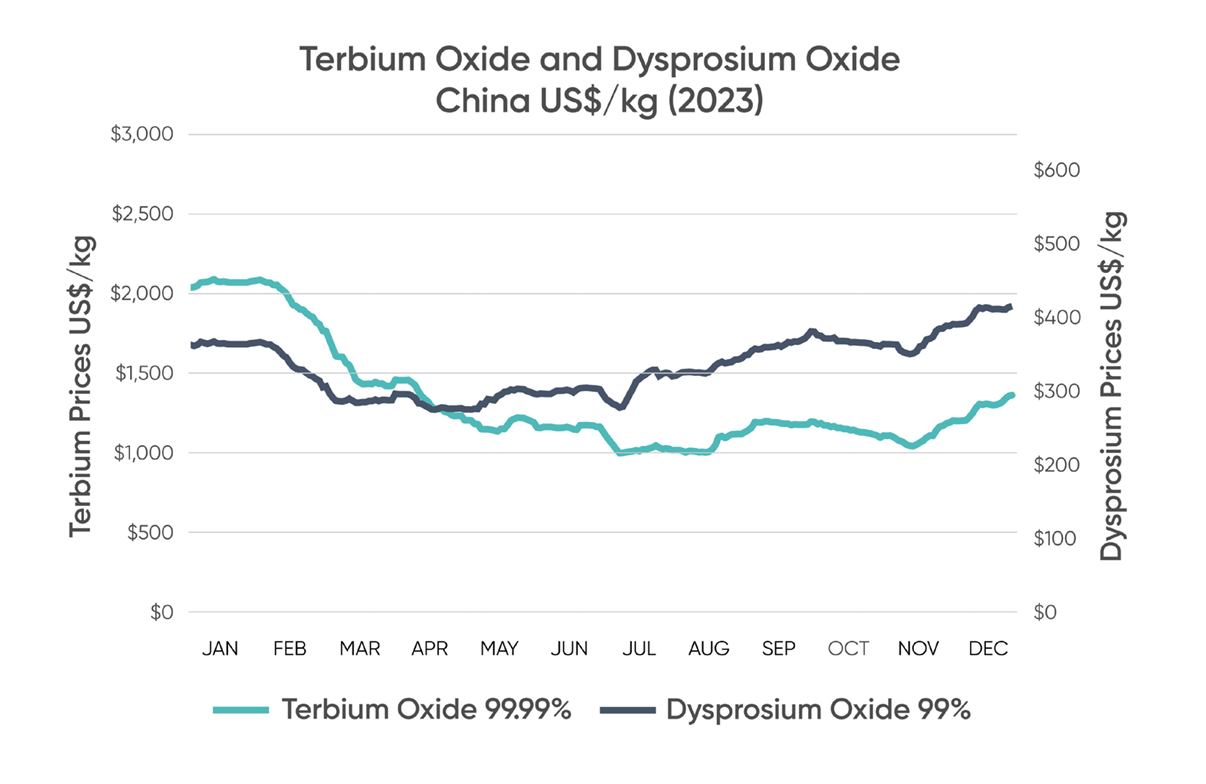
Source: Adamas Intelligence: Rare Earth Magnet Market Outlook to 2035 at Q2 202 The Ministry of Industry and Information Technology of the People’s Republic of China (MIIT), www.miit.gov.cn.
https://projectblue.com/blue/news-analysis/616/china%E2%80%99s-ministry-of-industry-and-information-technology-releases-rare-earth-quota-for-h2-2023
https://www.reuters.com/article/china-economy-trade-rareearths-idUSKBN2Z00PS
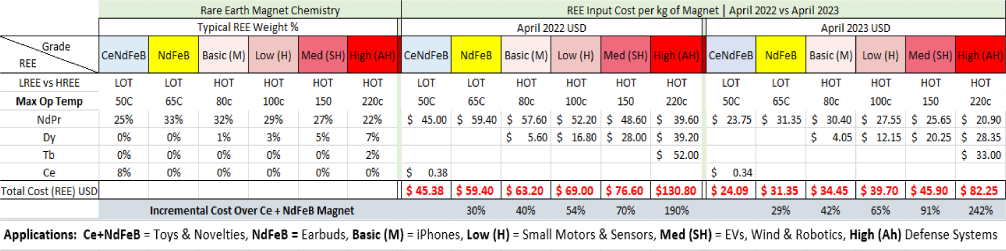
Rare Earth Inputs Prices For Various Grades Of Ndfeb Magnet
As detailed in the Table below, the cost difference between Ce+NdFeB and NdFeB LOT magnets vs HOT magnets increases rapidly.